 |
| Topic: | Eigenvalue problem basics |
| Post date: | May 29, 2024 |
| Due date: | May 30, 2024 |
The final linear algebra problem investigated in the course is finding invariant directions for a homogeneous linear mapping , , known as the eigenproblem
It is known that eigenvalues and associated eigenvectors exist to satisfy the above relation , , and the eigenproblem can be stated in matrix form as
Write the rotation matrix of angle around axis . Find the eigenvalues and eigenvectors of (1 point)
Find a matrix for which . What are the eigenvalues of ?(2 points)
If has eigenvalues 0,1,2 give values (or state that there is not enough information to specify a value) for:
rank
eigenvalues of
eigenvalues of
(3 points)
Determine the singular value decomposition and pseudo-inverse of a matrix (i.e., a row vector). (2 points)
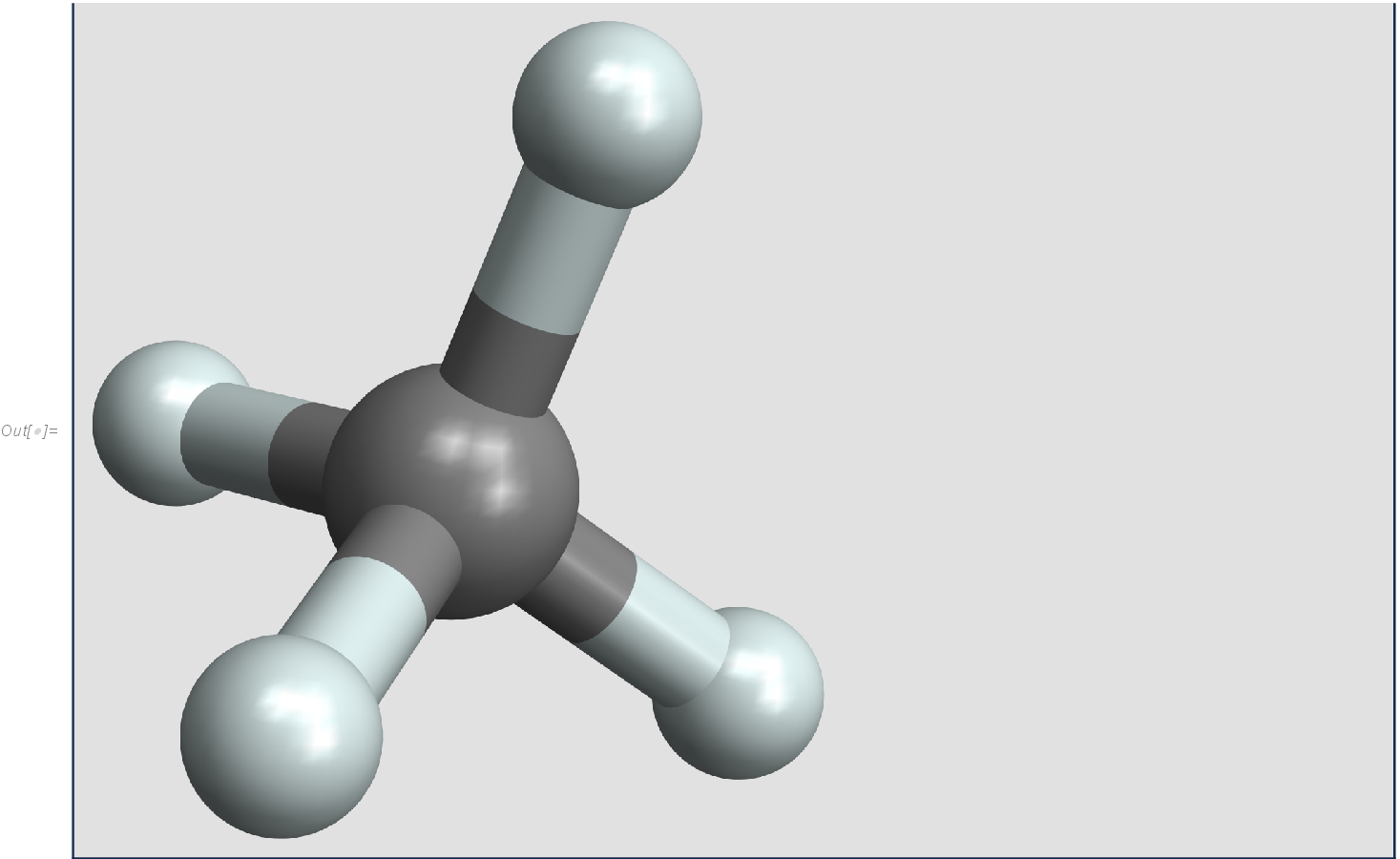
Molecules are composed from atoms interacting through electromagnetic forces generated by their electron orbital shells. The methane molecule contains a carbon atom and four hydrogen atoms
The positions of the methane atoms are given in Table 1.
|
||||||||||||||||||||||||
Molecular properties are largely determined by the vibration of the atoms around equilibrium positions. The equilibrium configuration of the methane molecule can be expressed through a single vector
with vectors from Table 1 for the carbon and four hydrogen atoms.
∴ |
Z=[0 0 0; -0.13066 -1.02319 0.359047; -0.713551 0.656267 0.503049; 1.01707 0.334488 0.215849; -0.172858 0.032433 -1.07795]; |
∴ |
fig=figure(); |
∴ |
ax = fig.add_subplot(projection="3d"); |
∴ |
ax.scatter(Z[1,1],Z[1,2],Z[1,3],color="black"); |
∴ |
ax.scatter(Z[2:5,1],Z[2:5,2],Z[2:5,3],color="red"); |
∴ |
ax.plot(Z[1:2,1],Z[1:2,2],Z[1:2,3],color="blue"); |
∴ |
ax.plot(Z[1:2:3,1],Z[1:2:3,2],Z[1:2:3,3],color="blue"); |
∴ |
ax.plot(Z[1:3:4,1],Z[1:3:4,2],Z[1:3:4,3],color="blue"); |
∴ |
ax.plot(Z[1:4:5,1],Z[1:4:5,2],Z[1:4:5,3],color="blue"); |
∴ |
Vibration around this equilibrium position is described by a displacement vector such that the position of the atoms is . The displacement vectors are known as vibrational eigenmodes and are determined by the eigenproblem
The matrix is symmetric , captures chemical bond strengths between the atoms and has a block structure
with diagonal blocks for , and off-diagonal blocks if there is a bond between atoms , and if there is no bond. The inertia matrix is block diagonal
with , .
Form the matrices in Julia. Compute . Note that
Compute the eigenvalues of .
Compute the eigenvectors of .
Plot the vibrational modes by plotting the equilibrium positions and the vibration mode with arbitrarily chosen to ensure visibility.